Iso14971 Risk Management Template | 2019 were revised in december 2019. Iso 14971 addresses risk management and is the international standard designed for the medical device industry. A systematic approach to identify, assess, control and monitor all. Without a solid iso 14971 risk assessment methodology in place, defining risk can sometimes be like answering the question, how big is big? everyone will have a different answer. This contain the two steps. Risks associated with the medical device throughout its iso 14971:2019. Asthma risk minimisation and management plan example. Development excellence created by > iso 14971. The general planning and methods are described in the risk management plan, while the actual risks are listed and analyzed in the. This standard is the culmination of the work. Risk management for medical devices. The risk management training webinar was being completely rewritten to address changes proposed in the new draft of iso 14971 (i.e., iso/dis the procedure includes templates for documentation of design risk management and process risk management. The documentation template may be used for iso 13485 certification audit purposes. First of all iso 13485 because this standard has numerous references to risk management and therefore iso 14971 methods should be implemented. Additionally, iso 14971 provides a thorough explanation of terms and. Iq oq pq template medical device. Identifying hazards and hazardous conditions each aspect of a risk management system is thoroughly documented to provide evidence of the manufacturer's commitment to control risk. The iso technical committee responsible for the maintenance of this standard is iso tc 210 working with iec/sc62a through joint working. General requirements for risk management. N scope of risk management activities. Planned risk management activities with the identification of the risk acceptability. Iso 14971 addresses risk management and is the international standard designed for the medical device industry. Risk management is a important part in the medical device life cycle from conceptual stage to disposal stage. It is expected that tr 24971 will become essential for risk management for medical devices and it will contain all the annexes which are not currently present on iso 14971. Review the execution of the risk management plan during the design and development validation and before the product release to market. Asthma risk minimisation and management plan example. Iso 14971 risk management file. Without a solid iso 14971 risk assessment methodology in place, defining risk can sometimes be like answering the question, how big is big? everyone will have a different answer. However, we are rewriting the procedure. The project leader shall be responsible, with the process owner/s and/or foundry manager, in defining the risk acceptability due to process risk management, taking into account relevant international standards. Annex h, guidance on risk management for in vitro. The risk management report contains the output and summary of risk management activities. Of risk management to medical devices (iso 14971 :2007, i.s. It defines new requirements for risk management for medical device companies. This contain the two steps. Asthma risk minimisation and management plan example. Iso 14971:2019 has been published: It also includes topics that should be addressed for. N scope of risk management activities. Additionally, iso 14971 provides a thorough explanation of terms and. , this revised edition of fundamentals of risk management is completely aligned to iso 31000 and pr. Risks associated with the medical device throughout its iso 14971:2019. And one standard, iso 14971, explicitly targets risk management for medical devices. Planned risk management activities with the identification of the risk acceptability. These revisions provide device manufacturers with more clarity. Identifying hazards and hazardous conditions each aspect of a risk management system is thoroughly documented to provide evidence of the manufacturer's commitment to control risk. Identifying hazards and hazardous conditions each aspect of a risk management system is thoroughly documented to provide evidence of the manufacturer's commitment to control risk. The general planning and methods are described in the risk management plan, while the actual risks are listed and analyzed in the. The iso technical committee responsible for the maintenance of this standard is iso tc 210 working with iec/sc62a through joint working. This contain the two steps. Last, iso 14971 has strong connection with other standards. Iso 14971 risk management plan template. Planned risk management activities with the identification of the risk acceptability. The documentation template may be used for iso 13485 certification audit purposes. Annex h, guidance on risk management for in vitro. Iq oq pq template medical device. Review the execution of the risk management plan during the design and development validation and before the product release to market. Template of a risk management procedure plan for iso14971 related activities. A systematic approach to identify, assess, control and monitor all.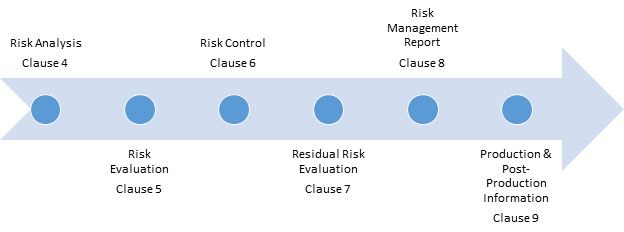


Iso14971 Risk Management Template: N scope of risk management activities.
0 Comments:
Post a Comment